Study of Chromista and Protozoa in a Hotspot Area at the Mediterranean Coast with Special Reference to the Potentiality to Use It as Bio-indicators 
2 National Institute of Oceanography and Fisheries, Alexandria- Egypt
3 Institute of Graduate Studies and Research, Department of Environmental Studies, Alexandria- Egypt
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2016, Vol. 6, No. 53 doi: 10.5376/ijms.2016.06.0053
Received: 07 Nov., 2016 Accepted: 12 Dec., 2016 Published: 12 Dec., 2016
Abo-Taleb H.A et al., 2016, Study of Chromista and Protozoa in a Hotspot area at the Mediterranean Coast with Special Reference to the Potentiality to Use It as Bio-indicators, International Journal of Marine Science, 6(53): 1-17 (doi:10.5376/ijms.2016.06.0053)
Chromista and Protozoa ranked the 2nd dominant group in the study area represent together 16.1 % of the total zooplankton with annual average of 1440 organisms/m3 and expressed by 69 species. Distinct differences that appeared in the dynamics, patterns of occurrence and numbers of the species in these locations were attributed to differences in physical and chemical conditions.Several environmental conditions appear to control the regional and seasonal distribution of such organisms. Ballast water plays an important role in species mechanical transmission through shipping movements. In conclusion, the community composition of the two phyla and their significant dominance indicated that, El-Mex Bay is a highly eutrophic system which shows signs of partial pollution. Accordingly, it is recommended that the waste water should be treated and/ or recycled before discharge into this natural aquatic system. It was important to produce monograph inside this work containing the photos of the organisms.
Introduction
In aquatic environments, zooplankton plays an important role in the transfer of energy from the primary producers to the higher level in the food chain (Nour El-Din, 1987). Furthermore, they are themselves favorite food items for many animals including economic fishes (El-Rashidy, 1987).
Protozoa and Chromista have a cosmopolitan distribution and play an integral role in the decomposition of organic matter, in nutrient cycling and in the maintenance of energy flow within both terrestrial and aquatic ecosystems (Anderson, 1988; Atkins et al., 2000). They can be of low abundance but widespread in environments that only marginally suit their survival or can rapidly colonize and exploit microenvironments that more optimally satisfy their needs (Anderson, 1988). Although relatively little is known about the potential ecological significance of protozoan assemblages in hydrothermal vent environments, many protozoa can tolerate reducing conditions and are likely candidates for survival in these marine ecosystems (Kouris, et al., 2007).
Several studies on zooplankton abundance, composition and seasonal variations have been carried out in the coastal water of Alexandria. Such as El-Maghraby and Halim (1965); Dowidar and El-Maghraby (1970a, b); AboulEzz, (1975); El-Zawawy, (1980); Dowidar et al., (1983); Khalil et al., (1983); AboulEzz et al., (1990); AboulEzz and Zaghloul (1990); and Abdel Aziz, (1997).
The near shore waters west of Alexandria have attracted the attention of some investigators such as Hussein (1997), who studied the zooplankton standing stock and community structure in relation to the impact of waste discharge in El-El-Mex Bay and Abdel Aziz (2000) studied zooplankton community at El-Dekhelah Harbor. The impact of water circulation and discharged wastes on zooplankton dynamics in the Western Harbor of Alexandria have been studied by Abdel Aziz (2002). She also recorded the short term variations of zooplankton community in El- Noubaria Canal (Abdel Aziz, 2005).
The water characteristics, phytoplankton and zooplankton population of El-Mex bay and El-Umoum drain were previously studied (Soliman and Gharib, 1998; Gharib, 1998; El-Sherif, 2006; Hussein and Gharib, 2012) and showed that, the continuous discharging polluted water into the bay caused massive development of algal blooms and a gradual deterioration of water quality created. Also, (Zakaria et al., 2007) illustrate the influence of salinity variations on the abundance and community structure of zooplankton in El-Mex Bay waters.
Protozoon plankton constitutes a significant proportion of total zooplankton biomass in a variety of aquatic environments (Mazumder et al., 1990). Studies of the distributional patterns of these smaller consumers and their spatial and temporal relationships with major hydrological features along the coastal water of Alexandria were studied by Hussein, 1977; Dowidaret al., 1983; AboulEzzet al., 1990; Abdel Aziz, 1997; Abdel Aziz et al., 2011; Hussein, 1997; Abo-Taleb, 2010, 2014; Abou Zaid et al., 2014; AboulEzz et al., 2014.
The present work is aims to analysis the species composition of Chromista and protozoa and their abundance along El-Mex Bay and to assess the impact of different environmental factors on their distributional pattern, in order to potentially of using it as bio-indicator on the environmental conditions.
Methodology
Research Area
El-Mex Bay is multi-polluted area, is elliptical in shape and it is located west of Alexandria. It extend for about 15 Km., the Bay has a mean depth of 10 m. Its surface area is about 19.4 Km2, and its volume 190.3 x 106 m3. The shoreline of El-Mex Bay is rocky with narrow sandy beaches. It receives a heavy load of wastewater both directly from industrial outfalls and indirectly from Lake Mariut via El-Mex Pumping Station. It lies about one kilometer upstream on El-Umoum Drain canal and pumping about 2.6 million m3/ year (Abdel-Halim, 2004). This is mainly agricultural drainage water collected by El-Umoum Drain, and comprises the overflow from LakeMariut. LakeMariut receives wastewater from the four sources in its eastern section, consisting of domestic, industrial and agricultural wastes. This liquid wastes fill the lake and overflow to the El-Umoum Drain and discharged to the sea via El-MexPumping Station.
El-Mex district is an industrial zone west of Alexandria city. As a consequence of growing heavy industries (chloro-alkali plant, petrochemicals, pulp, metal plating, industrial dyes, and textiles) and uncontrolled disposal of resulting wastes, in addition coastal water of El-Mex Bay received huge amounts of untreated industrial wastes.
Sampling
From autumn 2011 to autumn 2012, zooplankton samples were collected at eight different stations (Figure 1). Samples were collected vertically by using a standard plankton net (25 µm mesh size), lowered near the bottom up to the water surface. The collected fauna were fixed in 5% formalin solution. Protozoa were identified till the species level; zooplankton samples were brought to the lab and split into three random concentrated sub-samples of 5 ml for counting standing crop (organisms/m3). Along with Protozoa, temperature was measured and water samples were tested for salinity, phytoplankton biomass (Chlorophyll-a) was measured according to procedures described by Strickland and Parsons (1972) and dissolved oxygen (Strickland and Parsons, 1972).
Identification of different Protozoa species was carried out according to Jörgensen, (1924), Tregouboff and Rose (1957), Marshall, 1969, Paulmier, (1997), and WORMS's database.
All collected data in the present study were tabulated and appropriate graphs are constructed. The data were subjected to the statistical treatment to find biological indices. The Correlation coefficient (r) and multiple regression analysis were computed using MINITAP 14 program for rotifers with the ecological measured parameters and chlorophyll-a concentration at p ≤ 0.05.
.png) Figure 1 El-Mex Bay coastal area showing sampling stations during the investigation period |
Results
Hydrographic conditions
El-Mex Bay seemed to be impacted by various land-based sources and man-made activities. Near shore stations were influenced by the discharge from El- Umoum Drain as well as water flowing from the WesternHarbor. Low salinities (reaching 10.82 ‰ during winter) were observed inshore, matching the high flow period of brackish water from LakeMariut. Oxygen levels declined to 5.6 mg/1 due to the anoxic nature of the lake water. The discharge of nutrients (nitrates and phosphates) rendered the bay a eutrophic system, with chlorophyll-a levels varying between 10.14 µg/l in spring 2012 and 34.38 µg/lin summer 2012 (Figure 2).
.png) Figure 2 Water quality characteristics for different seasons collected along coastal water of El-Mex Ba |
Temporal distribution and diversity of the organisms
During the study period, Chromista and Protozoa contributed 16.1% of Total zooplankton in El-Mex Bay with average of 1440 organisms/m3. The total number of species recorded in the study area was 69 species (Table 1) divided into four major phyla belonging to 4 classes, 9 orders, and 22 families under 32 genera. The four phyla were: Ciliophora, represented by 2 classes, 2 orders, 12 families, 20 genera, and 49 species. Amoebozoa represented by 1 class, 1 order, 2 families, 2 genera, and 5 species. Sarcomastigophora represented by 2 genera and 2 species. Foraminifera represented by 1 class, 6 orders, 8 families, 8 genera, and 12 species (Table 1 and Plates I to V). Table 1 represented the most dominant species (relatively 43% of the total organisms).
.png) Table 1 List of studied species |
Tintinnopsisberoidea, Favellaehrenbergi represented the most dominant species formed 9.1 %, 6.9 % respectively of total counts, followed by Difflugiaoblonga, Favellapanamensis and Favellaazorica which represented 3.1 % for each (Table 2).
Their counts ranged between maximum of 5860 organisms/m3 at station VII during spring and minimum of 212 organisms/m3 at station V during autumn 2011. Generally, winter and spring seasons showed maximum densities (average of 2272 and 2045 organisms/m3). On the other hand, minimum average counts 519 organisms/m3 were recorded at the beginning of the study during autumn 2011 (Table 3).
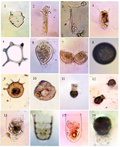 Plate I (1)Amphorellaurceolata, (2)Amphorellopsisacuta, (3)Amphorides amphora (4)Amphorides minor, (5)Archiriscushertwigi, (6 and 7)Euplotessp., (8)Myxosphaeracoerulea(9) Centropyxis aculeate, (10)Centropyxisecornis(11)Codonellopsismorchella, (12)Codonellopsisovata, (13)Coxliellabolivari, (14)Coxliellalaciniosa, (15)Coxliella longa and (16)Difflogiaurceolata. |
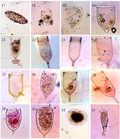 Plate II (17)Difflugiaoblonga, (18)Epiplocylisundella, (19)Favellaadriatica,(20)Favellaazorica, (21- 24)Favellaehrenbergi, (25) Favella campanula, (26)Favellafranciscana,(27 and 28)Favellamarkusouzkyi, (29)Favellapanamensis, (30) Favellaserrata, (31)Difflugiaglobulosa and (32)Rhabdonellaapophysata. |
 Plate III (33)Helicostomellasubulata, (34)Helicostomellaedentata, (35 and 36)Metacylisannulifera,(37 and 38)Undellahyalina, (39)Undellopsismarsupialis, (40)Eutintinnuselongatus, (41)Eutintinnusfraknoii,(42) Eutintinnusapertus(43 and 44) Eutintinnuslusus-undae, (45 and 46) Leprotintinnusnordqvistii, (47)Tinnopsis radixand(48)Tintinnopsis acuminate; |
 Plate IV (49)Tintinnopsisberoidea, (50)Tintinnopsisgracilis, (51)Tintinnopsis campanula,(52)Tintinnopsiscompressa, (53)Tintinnopsiscorniger, (54)Tintinnopsisfracta, (55)Tintinnopsiskofoidi,(56) Tintinnopsislobiancoi(57)Tintinnopsis major, (58)Tintinnopsisplagiostoma, (47)tinnopsis cylindrica, and(60)Tintinnopsisdavidoffi |
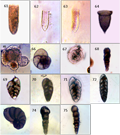 Plate V (61)Tintinnopsiskarajacensis, (62 and 63)Proplectellaparva, (64)Cyttarocylis cassis, (65 and 66)Globigerina inflata, (67)Ammodiscussemiconstrictus, (68)Bigenerinatextularioidea, (69)Bolivinainflata,(70)Bolivinalaevigata, (71)Bolivinarobusta, (72)Bolivinavariabilis, (73)Nonionoidesgrateloupi, (74)Reophaxnorthviewensisand (75)Trepeilopsisspiralis. |
.png) Table 2 The most dominant species (organisms/m3) and their percentages to total organisms |
.png) Table 3 Total counts (organisms/m3) at different stations during the study period |
Spatial distributions
Data presented in Table (4) and Figure (3) showed that station VI during autumn 2012 has the highest number of species; being 27 species where salinity recorded high value 28.53‰. Followed by station III during winter and autumn 2012 (24 and 20 species) respectively. On the other hand, the lowest number of species (four species) was recorded at station I during autumn 2011 and summer 2012 and at stations I and IV.
.png) Figure 3 The species density at studied stations during different seasons (organisms/m3) |
Data in Table (5) and Figure (3) showed that, the highest density was recorded at station VII during spring 2012 (5860 organisms/m3). On the other hand, the lowest density was recorded at station V during autumn 2011 (212 organisms/m3).
.png) Table 4 Spatial diversity (Number of species) during the study period |
.png) Table 5 Spatial distribution (organisms/m3) during the study period |
The spatial distribution of species through all the study period (Figure 4) showed that the highest density was recorded at station VII (2679 organisms/m3) with 33 species, followed by station IV recorded 1776 organisms/m3 and minimum diversity being 24 species. Station VI showed maximum number of species 47 species with total number 1516 organisms/m3. On the other hand, the lowest station density was V (715 organisms/m3) with 30 species followed by station II 845 organisms/m3 and 31 species.
.png) Figure 4 Spatial distribution of organisms at the study area |
Temporal variations
The highest number of species was recorded during winter 2012 being 43 species (2272 organisms/m3). On the other hand, autumn 2011 and summer 2012 represented the lowest counts (519 and 773 organisms/m3) respectively. The remaining seasons (spring and autumn 2012) recorded 40 species (2045organisms/m3) and 38 species (1588organisms/m3) respectively (Figure 5).
Frequency of occurrence
Results of occurrence and distribution of the species are displayed in Figure (6). The occurrence of species was varied from season to another during the entire study period where, the highest percentage of occurrence 100% (recorded in the five seasons) was seven species; Centropyxisaculeata, Difflugiaoblonga, Favellaazorica, Tintinnopsisberoidea, T. campanula, T. cylindrica, and T. lobiancoi. On contrast, the lowest percentage of occurrence (20%) recorded for 11 species that represented only during one season; Amphorellopsisacuta, Amphorides amphora, A. minor, Coxliellalaciniosa, C. longa, Helicostomellaedentata, Metacylisannulifera, Paraundellacaudata, Tintinnopsis acuminate, T. rotundata and T. subacuta,. Twelve species were occurred to represent 80% occurrence of the study period (recorded during four seasons) they were; Difflogiaurceolata, Eutintinnusapertus, E. elongatus, E.fraknoii, Favellaehrenbergi ,F. serrata , Tintinnopsisfracta , T. radix , Adelosinaelegans, Globigerina bulloides, G. inflata , and Archiriscushertwigi . The remaining 37 species are divided into 16 species occurred in three seasons represent 60% of the study period and 21 species appeared in two seasons with occurrence 40% of the study period.
.png) Figure 5 Temporal variations in Chromista and Protozoa species |
.png) Figure 6 Histogram showing the frequency of occurrence of the species during different season |
The spatial distribution of data represented in (Figure 7) showed that 8 species occurred at all stations; Difflugiaoblonga, Favellaazorica, F. ehrenbergi,F. panamensis,F. serrata,Tintinnopsisberoidea, T. campanula, and T. radix. In contrast, 6 species (represents 12.5%) were restricted to one station only Amphorides amphora, A. minor, Coxliellalaciniosa, Tintinnopsis acuminate, T. rotundataandUndellopsismarsupialis. On the other hand, 11 species were recorded at two stations with occurrence 25% of all study areaAmphorellopsisacuta, Codenelladaday, Coxliellabolivari, C. longa, Helicostomellaedentata, Paraundella caudate, Tintinnopsiskofoidi, T. subacuta, Ammodiscussemiconstrictus, Bolivinavariabilis, and Nonionoidesgrateloupi.
.png) Figure 7 Histogram showing the frequency of occurrence of the species at different season |
Concerning to the occurrence of 37.5% eighteen species were found at three stations; Amphorellaurceolata, Centropyxisecornis, Codonellopsisovata, Cyttarocylis cassis, Epiplocylisundella, Favella campanula, F. franciscana, Helicostomellasubulata, Proplectellaparva,Rhabdonellaapophysata, Tintinnopsisfracta , T. gracilis , T. plagiostoma , T. karajacensis, Bolivinainflata , B. laevigata , Reophaxnothviewensis, and Trepeilopsisspiralis.
Eight species were recorded at four stations (50%) namely; Difflugiaglobulosa, D. urceolata, Eutintinnuselongatus, E. fraknoii, E. lusus-undae, Metacylisannulifera, Bolivinarobusta, and Globigerina bulloides.
Six species occurred at five stations with percentage 62.5%; Eutintinnusapertus, Favellamarkzowski, Tintinnopsiscompressa, T. corniger ,T. lobiancoi , and Bigenerinatextularioidea . Whereas, 5 species occurred with 75 % of the study area at 6 stations; Euplotes sp., Favellaadriatica, Tintinnopsisdavidoffi, T. major and Adelosinaelegans. On the other hand, 5 species were recorded at 7 stations; Centropyxisaculeata, Leprotintinnusnordqvistii, Tintinnopsis cylindrica, Undellahyalina, and Globigerina inflata with occurrence 87.5%.
Correlation analysis of total species versus the ecological condition
At P ≤ 0.05, Total species numbers inversely correlated with depth during winter 2012 and spring 2012 with "r" values were 0.645 and 0.761 respectively to the two seasons, during summer 2012 total species count were correlated negatively with water temperature and nitrate concentrations NO3 (µg/l) at "r" values 0.839 and 0.856 respectively to the two parameters.
On the other hand during autumn 2012 the species densities showed indirect correlation with silicate concentrations SiO4 (mg) in the bay water where "r" value was 0.012.
Multiple regression analysis (Prediction equations):
Regression equations (R.E.) constructed for estimating the relationships between standing crop and the all measured environmental factors. The resulted prediction equations for estimating the total number of studied plankton species (belonging to the two phyla), gives results have some deviation from the exact realnumber; this error can be minimized by using stepwise regression analysis (S.R.E.). The stepwise regression analysis was performed to exclude parameters that were not strongly correlated (insignificantly) with total species counts (p>0.05) from the equations.
Multiple regression analysis of total species count versus visibility, depth, salinity (‰), pH, temp., DO (mg/l) and chlorophyll-a concentrations (µg/l) result in the following equations (Table 6).
.png) Table 6 Regression and stepwise regression equations during the studied seasons |
Cluster Analysis (Similarity index):
The result of cluster analysis using MINITAB Release 14 computer programs based on seasons and stations data including the physico-chemical parameters and zooplankton composition are illustrated in the following dendrograms.
Similarity analysis
Two major clusters were constructed between different seasons, with only 63.3% similarity, the first cluster separated winter and spring 2012 with the highest similarity, being 98.8%. The second cluster is divided into two sub-clusters with similarity 89.4%, the first sub-cluster separate autumn 2012 and the second sub-cluster contained the remaining two seasons autumn 2011 and autumn 2012 with 89.4% similarity between them (Figure 8).
The analysis of similarity between the studied stations illustrated in Figure (9) which showed that, locations were divided into two major clusters with 75.37% similarity, the first cluster divided into two sub-clusters with 94.74% similarity, The first sub-cluster separates station VIII while the second included stations IV and VII with similarity 96.96%. On the other hand, the second cluster was divided into two sub-clusters with 88.81% similarity; the first one includes station V while the second contained the remaining four stations. The highest similarity was 97.52% between stations I and III.
.png) Figure 8 Dendrogram showing the similarity between different seasons according to correlation coefficient and complete linkage depending on the studied species and environmental parameter |
.png) Figure 9 Dendrogram showing the similarity between different stations according to correlation coefficient and complete linkage depending on the studied species and environmental parameters. |
Discussion
Chromista and Protozoa occupied together the 2nd ranking of abundance among zooplankton groups in El-Mex Bay contribution 15.6% of the total zooplankton counts (average of 1440 organisms/m3), dominated by ciliates. The two phyla are characterized by many specific features in there structure and function, present an important ecological assemblage in aquatic ecosystem and play a critical and crucial role in the function of microbial food webs in order to their role as bio-indicators of water quality (Xuet al., 2008). Zakaria et al., 2007 found the influence of salinity variations on zooplankton community in El-Mex Bay and stated that Chromista and Protozoa were the second important groups after rotifers in the bay.
In the investigated area, the optimum temperature for flourishing of these two phyla ranged between 14.39- 30.68 °C and the optimum salinity from 10.82- 28.53‰ which agreed with (Abo-Taleb et al., 2014; Hendy, 2013) whom found temperature ranged between 16.22 and 31.2 °C and salinity between 15.32 and 30.34‰ were favorable conditions for growth and high abundance of zooplankton in the bay. On the other hand, low temperature (during winter 2012) and salinity were unfavorable for the development of Chromista and protozoa assemblages.
Several environmental conditions appear to control the regional and seasonal distribution of the two phyla including the prevailing physico-chemical conditions like temperature and salinity (Smetacek, 1981; Sanders, 1987). Many authors observed that the maximum Chromista and protozoa abundance is associated with high salinity (Verity, 1987).
The water column of El-MexBay suffers from pronounced turbidity, particularly in front of the land runoff, whereas the Secchi-disc readings were mostly < 1 m. Such turbidity is attributed to the strong mixing caused by discharged wastes, high eutrophication conditions, heavy traffic of fishing boats, and high count of plankton organisms. However, the open area of the bay shows comparatively high in transparency (up to 5 m). These observations agreed with (Mahmoud, et al., 2009, Dorgham, 2011; Hendy, 2013).
Chlorophyll-a is considered as essential component responsible for photosynthesis process. It was primary photosynthesis pigment in all oxygen evolving photosynthetic plants. In the present study we used chlorophyll- a concentration as an indicator of phytoplankton abundance and biomass in coastal waters. In the present study the maximum chlorophyll- a concentrations recorded at station (I) 52.65 µg/l. The high concentration of Chl-a content recorded in the water is coincided with low salinity, high temperature and high values of nutrient salts, which reflects such eutrophication condition caused by drainage effluents. These data agreed with that obtained by (El- Sherif, 2006, Hendy, 2013 and Abo-Taleb et al.,2016) where Chl-a ranged from 9.4 μg/l to 21.3 μg/l. Gharib, 1998 observed that the phytoplankton abundance and the number of species increased consistently towards the outer region of El-Mex bay, where the salinity was low.
Chromista and Protozoa community in El-Mex Bay is pronounced affected by the dispersion pattern of discharged waters. Higher values were particularly observed during winter 2012 (2272 organisms/m3) while autumn 2011 displayed lower densities (519 organisms/m3). The species density reached the maximum values at station VII during spring 2012 (5860 organisms/m3) due to the predominance of Centropyxis aculeate, Difflugiaoblonga, Favellaazorica, Tintinnopsisberoidea, T. campanula, T. cylindrica, and T. lobiancoi.
El-Sherif (2006) stated that Chromista and Protozoa were the most highly diversified groups in the western part of Alexandria Mediterranean Coast. It was represented by 63 species (48.46% to the total number of the recorded zooplankton species) classified into, 40 tintinnid species, 11 foraminifern and 12 species of fresh water ciliates. All tintinnid species are marine forms while some of Foraminifera species are belonging to freshwater forms such as Elphidium sp., Ammonia beccarri and Quinquliculina sp. The pronounced occurrence of these species as well as the freshwater ciliates could be considered as indicators of the freshwater discharge to the area. El-MexBay has the highest tintinnid densities during the study period which was dominated by Tintinnopsis beroidea and this also agreed with Nour El- Din, 2001.
Ciliated Protozoa are considered as bio-indicators. The absence of these organisms indicates the presence of toxic substances, such as phenols, cyanids, and heavy metals. The presence of these organisms indicates oxygen deficiency, system overload, and putrefaction. An increased number of several different bacteria, the presence of Cyanophyta, Zooflagellata, and Ciliata are an indication of water overloaded with organic matter, i.e. an indication of polysaprobic processes and oxygen deficiency (Németh-Katona, 2008).Some Protozoan species are considered as indicator of the pollution with sewage pathogenssuch as the genera Euplotes, Centropyxis and Difflugia. These freshwater species that encountered in this study confirmed the contamination of the by sewage, this agree with Froneman, 2004 who mentioned that, The presence of protozoan freshwater species in marine coastal areas are considered a biomarkers on the presence of fresh water discharge into these areas, and according to types of this species can determine the source of water discharged neither rivers, lakes, drainage or sewage.
All the results of the studies indicating that potentiality of zooplankton as bio-indicator are very high and in some countries they confirmed these concepts to monitor the water quality (Ferdous and Muktadir, 2009). The numbers of freshwater species that presented in this investigation were considered as bio-indicator on the huge amount of freshwater discharged into the study area, also the great numbers of ciliated protozoa play as bio-indicators of pollution and indication of water quality declining.
Some species known to be characterize the clear open deep water like Archiriscushertwigi, Myxosphaeracoerulea, these two species were encountered at the bay during this study and doesn’t recorded before from the shallow water of Egyptian Mediterranean Coast. After investigations, there is only one reason supposed to discuss this phenomena, it is the mechanical transmission through the ballast water. It is well known fact that, El-Mex Bay lied between two of the biggest harbors at the Egyptian coasts, many ships transport some heavy industrials like metals includes irons products and others, these ships discharge the ballast water directly into the bay before there entrance to the harbors including many invading organisms from different regions and origins. Ballast water is considered one of the primary transport vectors for the transfer in order to introduction of non-indigenous zooplankton (DiBacco et al., 2012). It may be also a result of the prevailing northeastern current at the Egyptian Mediterranean Coast that can bring the open water organisms into the shallow areas.
Conclusion
Chromista and Protozoa was the most diver groups in the bay represented by 69 species and formed 34% of total zooplankton groups in El-Mex Bay. The most dominant forms were; Centropyxis aculeate, Difflugiaoblonga, Favellaazorica, Tintinnopsisberoidea, Tintinnopsis campanula, Tintinnopsis cylindrica, and Tintinnopsislobiancoi.
Zooplankton community in El-Mex Bay is pronounced affected by the dispersion pattern of discharged waters. The water masses in the bay showed different communities, relative to the salinity differences. Higher values were particularly observed during winter 2012 (2272 organisms/m3) while autumn 2011 displayed lower densities (519 organisms/m3). The species belonging to the two studied phyla reached their maximum densities at station VII during spring 2012 (5860 organisms/m3) due to the predominance of Centropyxis aculeate, Difflugiaoblonga, Favellaazorica, Tintinnopsisberoidea, T. campanula, T. cylindrica, and T. lobiancoi. These species were important contributors to the tintinnids populations at most stations in different seasons. The salinity is the limiting factor in the distribution of Chromista and protozoa, where the maximum abundance is associated with high salinity. Some species cane invades into new regions through the ballast water.
In conclusion, the community composition of protozoa and their significant dominance indicated that, El-Mex Bay is a highly eutrophic system which shows signs of partial pollution and this coincide with the results of El-Raey et al., 2015 recorded high concentrations of nutrient salts and prevailing eutrophication conditions. Accordingly, it is recommended that the waste water should be treated and/ or recycled before discharge into this natural aquatic body.
Abdel Aziz N.E.M., 1997, Zooplankton production along Egyptian Mediterranean coast of Alexandria, with special references to life history of one copepod species, Ph.D. Thesis, Faculty of Science, Mansoura University, 384 pp.
Abdel Aziz N.E.M., 2000, Weekly observations on zooplankton community in a sea-lake connection (Boughaz El- Maadiya), Egyptian Bulletin of Faculty of Science, Alexandria University, 40 (1, 2): 68-83.
Abdel Aziz N.E.M., 2002, Impact of water circulation and discharge wastes on zooplankton dynamics in the Western Harbor of Alexandria, Egypt, Egyptian Journal of Aquatic Biolology and Fisheries, 6 (1): 1- 21.
Abdel Aziz N.E.M., 2005, Short term variations of zooplankton community in the Western El- Noubaria Canal, Egypt, Egyptian Journal of Aquatic Research, 31 (1): 119- 132.
Abdel Aziz N.E.M., Aboul Ezz S.M., Abou Zaid M.M., and Abo-Taleb H.A., 2011, Temporal and spatial dynamics of rotifers in Rosetta Estuary, Egypt, Egyptian Journal of Aquatic Research, 37 (1): 59- 70.
Abdel-Halim A.M., 2004, Chemical equilibria studies of some phosphorus compounds under different conditions, Ph.D. Thesis, Faculty of Science, Alexandria University, Egypt 260 pp.
Abo-Taleb H.A., 2010, Dynamics of zooplankton community in the connection between the Mediterranean Sea and the River Nile at Rosetta Branch, Egypt, M.Sc. Thesis, Al-Azhar University, Faculty of Science, 183 pp.
Abo-Taleb H.A., 2014, Plankton as an indicator of environmental changes in El-Mex Bay of the Mediterranean Coast, Egypt, Ph.D. Thesis, Al-Azhar University, Faculty of Science, 267 pp.
Abo-Taleb H.A., Aboul Ezz S.M., Abdel Aziz N.E., Abou Zaid M.M. and El Raey M., 2016, Detecting Marine Environmental Pollution by Biological Beacons and GIS program, Journal of FisheriesSciences.com, 10 (4): 069-083.
Aboul Ezz S.M., 1975, A quantitative and qualitative study of zooplankton in Alexandria region with special reference to Appendicularia, M. Sc. Thesis, Faculty of Science, AlexandriaUniversity, 234 pp.
Aboul Ezz S.M., Hussein M.M. and Sallam N.A., 1990, Effect of domestic sewage discharge on the distribution of zooplankton organisms in the Eastern Harbour of Alexandria (Egypt), Bulletin of High Institute of Public Health, 20 (4): 861- 874.
Aboul Ezz S.M., and Zaghloul F.A., 1990, Phytoplankton-zooplankton relationship in the surface water of the Eastern Harbour (Alexandria), Bulletin of National Institute of Oceanography and Fisheries, 16 (1):19-26.
Aboul Ezz S.M., Abdel-Aziz N.E., Abou Zaid M.M., El Raey M., and Abo-Taleb H.A., 2014, Environmental assessment of El-Mex Bay, Southeastern Mediterranean by using Rotifera as a plankton bio-indicator, Egyptian Journal of Aquatic Research, 40: 43- 57.
https://doi.org/10.1016/j.ejar.2014.03.005
Abou Zaid M.M., El Raey M., Aboul Ezz S.M., Abdel-Aziz N.E. and Abo-Taleb H.A., 2014, Diversity of Copepoda in a Stressed EutrophicBay (El-Mex Bay), Alexandria, Egypt, Egyptian Journal of Aquatic Research, 40: 143-162.
https://doi.org/10.1016/j.ejar.2014.05.001
Anderson R.O., 1988, Comparative Protozoology.Ecology, Physiology, Life History, Springer Verlag, New York 433pp.
https://doi.org/10.1007/978-3-662-11340-0
Atkins M.S., Teske A.P., and Anderson R.O., 2000, A survey of flagellate diversity at four deep-sea hydrothermal vents in the eastern Pacific Ocean using structural and molecular approaches, Journal of Eukaryotic Microbiology, 47: 400-411.
https://doi.org/10.1111/j.1550-7408.2000.tb00067.x
DiBacco C., Humphrey D.B., Nesmith L.E. and Levings C.D., 2012, Ballast water transport of non-indigenous zooplankton to Canadian ports, ICES Journal of Mararine Science, 69 (3): 483-491.
https://doi.org/10.1093/icesjms/fsr133
Dorgham M.M., 2011, Eutrophication problem in Egypt. Book: Eutrophication: causes, consequences and control, Chapter 8: 171-194.
Dowidar N.M., Khalil A.N., El-Maghraby A.M., and El-Zawawy D.A., 1983, Zooplankton composition of the Eastern Harbour of Alexandria Egypt. Rapport Commissione Internationale Mer Méditerranée, 28 (9): 195-199.
Dowidar N.M. and El-Maghraby A.M., 1970a, The neritic zooplankton of South Mediterranean at Alexandria. Part I- Distribution and Ecology of the zooplankton organisms with special reference to Copepoda, Bulletin of Institute of Oceanography and Fisheries, 1: 225-273.
Dowidar N.M. and El-Maghraby A.M., 1970b, Consideration of the total zooplankton community, Bulletin of Institute of Oceanography and Fisheries, 1: 275-303.
El-Maghraby A.M., and Halim Y., 1965, A quantitative and qualitative of the plankton of Alexandria waters, Hydrobiologia, 25 (1-2): 221 pp.
El-Raey M., Nasr S. and Hendy D.M., 2015, Integrating Knowledge to Assess Coastal Vulnerability to Sea-Level Rise In El-Mex Bay, Egypt: The Application of the DIVA Tool, International Journal of Marine Science, 5: 1-7.
El- Rashidy H.H., 1987, Ichtyoplankton of the south eastern Mediterranean Sea off the Egyptian Coast, M. Sc. Thesis, Faculty of Science, Alexandria University, 313 pp.
El- Sherif Z., 2006, Effects of Industrial, Touristic and Marine Transport on Physical, Chemical and Biological Characteristics of Water and Fish Populations, West of Alexandria, NIOF Report, A.R.E., 320pp.
El- Zawawy D.A., 1980, Seasonal variations in the composition and biomass of zooplankton community in the Eastern Harbor of Alexandria, M. Sc., Faculty of Science, AlexandriaUniversity, 220 pp.
Ferdous and Muktadir, 2009, A Review: Potentiality of Zooplankton as Bioindicator. American Journal of Applied Sciences, 6 (10): 1815-1819.
https://doi.org/10.3844/ajassp.2009.1815.1819
Froneman P.W., 2004, Zooplankton community structure and biomass in a southern African temporarily open/closed estuary, Estuarine, Coastal and Shelf Science, 60: 125- 132.
https://doi.org/10.1016/j.ecss.2003.12.002
Gharib S.M., 1998, Phytoplankton community structure in Mex Bay, Alexandria, Egypt, Egyptian Journal of Aquatic Biology and Fishes, 2, 81-104.
Hendy D.M., 2013, Assessing the coastal vulnerability to climate change in El- Max Bay, Egypt, M.Sc. Thesis, Institute for Graduate Studies & Research (IGSR), 122 pp.
Hussein M.M., 1977, A study of the zooplankton in the Mediterranean waters off the Egyptian Coast during 1970-1971 with special reference to copepods, M. Sc. Thesis, Faculty of Science, Alexandria University, 228 pp.
Hussein M.M., 1997, Distribution of zooplankton assemblages in El-Mex Bay, Alexandria, Egypt, National Institute of Oceanography and Fisheries, 23: 217-240.
Hussein N.R. and Gharib S.M., 2012, Studies on spatio-temporal dynamics of phytoplankton in El-Umum drain in west of Alexandria, Egypt. Journal of Environmental Biology 33: 101-105.
Jörgensen E., 1924, Mediterranean Tintinnidae, Report on the Danish oceanographical expeditions 1908-1910 to the Mediterranean and adjacent Seas, volume II. Biology, No. 8, J.3 (Thor Expedition), Copenhagen.
Khalil A.N., El-Maghraby A.M., Dowidar N.M., and El-Zawawy D.A., 1983, Seasonal variations of zooplankton biomass in the Eastern Harbour of Alexandria, Egypt. Rapport Commissione Internationale Mer Méditerranée, 28(9): 217-218.
Kouris A., Juniper S.K., Bourg G.F., and Gaill F.O., 2007, Protozoan–bacterial symbiosis in a deep-sea hydrothermal vent folliculinid ciliate (Folliculinopsis sp.) from the Juan de Fuca Ridge, Marine ecology, 28: 63- 71.
https://doi.org/10.1111/j.1439-0485.2006.00118.x
Mahmoud T.H., Masoud, M.S., Shaltout N.A., and Hussein N.R., 2009, The biological pump of carbon dioxide in El-Mex Bay, Alexandria, Egypt, Egyptian Journal of Aquatic Research, 36 (1): 11-20.
Marshall S.M., 1969, Fiches d’identification du zooplankton: Protozoa, Tintinnida. In: Fraser JH, Hansen VK (eds) Conseil Permanent International pour l’Explanation de la Mer, Charlottenlund slot- Denmark. Sheets 117-127
Mazumder A., McQueen O. J., Taylor W.O., Lean O.R.S. and Dickman M.D., 1990,. Micro-and mesozooplankton grazing on natural picoplankton and nanoplankton in contrasting plankton communities produced by planktivore manipulation and fertilization, Archiv Der. Hydrobiologie., 118: 257-282.
Németh-Katona J., 2008, The environmental significance of bioindicators in sewage treatment, ActaPolytechnica Hungarica, 5 (3): 117-124.
Nour El-Din N.M., 1987, Ecology and distribution of pelagic copepods in the Hutchin Son Educational Ltd., London, 244 pp.
Nour El-Din N.M., 2001, Ecological investigation of the tintinnid community along the coastal waters of Alexandria, Egypt, Egyptian Journal of Aquatic Biology and Fisheries, 5 (3): 165 -177.
Paulmier G., 1997, Tinninids (Ciliphora, Oligotrichida, Tintinnina) De L'Atlantique Boreal, De L'OceanIndienet de quelquesmersadjacentes: Mediterranean, Mercaraibe, Mer Rouge, Inventaireet Distribution. Observations baseessur les loricas, Rapport IFREMER DRV/RH /97-17, Brest, France.
Sanders R.W., 1987, Tintinnds and other microzooplankton seasonal distribution and relationships to resources and hydrography in a marine estuary, Journal of Plankton Research, 9: 65-77.
https://doi.org/10.1093/plankt/9.1.65
Smetacek V., 1981, The annual cycle of Protozooplankton in the Kiel Bight Marine Biology, 63: 1-11.
Soliman A.M. and Gharib S.M., 1998, Water characteristics, phytoplankton and zooplankton population of El-Mex bay region. Bulletin of Faculty of Science Alexandria University, 38: 45-66.
Strickland J.D.H. and Parsons T.R., 1972, A practical handbook of sea water analysis , second edition. Journal of the Fisheries Research Board of Canada, 176: 1-310.
Tregouboff G. and Rose M., 1957, Mannual de planktologie Mediterranean C.N.R.S., Paris, 208 pp.
Verity P.G., 1987, Abundance, community composition, size distribution and production rates of tintinnids in Narragansett Bay, Rhode Island, Estuarine, Coastal and Shelf Science, 24:671- 690.
https://doi.org/10.1016/0272-7714(87)90106-5
Xu H., Song W., Warren A., and Hu X., 2008, Plankton protist communities in a semi-enclosed mariculture pond: Structural variation and correlation with environmental condition, Journal of the marine Biological Association of the UK, 88: 1353- 1362.
https://doi.org/10.1017/S0025315408002129
Zakaria H.Y., Radwan A.A., and Said M.A., 2007, Influence of salinity variations on zooplankton community in El-MexBay, Alexandria, Egypt, Egyptian Journal Of Aquatic Research, 33 (3): 52- 67.
WORMS, www.world register of marine species.com
. PDF(1500KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Hamdy A. Abo-Taleb
. Nagwa E. Abdel Aziz
. Sawsan M. Aboul Ezz
. M. El Raey
. Mohamed M. Abou Zaid
Related articles
. Chromista
. Protozoa
. El-Mex Bay
. Eutrophic
. Bio-indicator
. Environmental conditions
Tools
. Email to a friend
. Post a comment


